
Formula Estructural Del Propano Acido
Take acetone (C 3 H 6 O) for example. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. This is an aprotic solvent because the highly electronegative atom, oxygen, is not bonded to a hydrogen atom. The hydrogen atoms are bonded to the carbon instead, which is a.

What Is The Molecular Formula Of Propanone
To Your Health: Acetone in Blood, Urine, and Breath. Acetone is formed in the human body as a by-product of lipid metabolism. Normally, acetone does not accumulate to an appreciable extent because it is oxidized to carbon dioxide and water. The normal concentration of acetone in the human body is less than 1 mg/100 mL of blood.
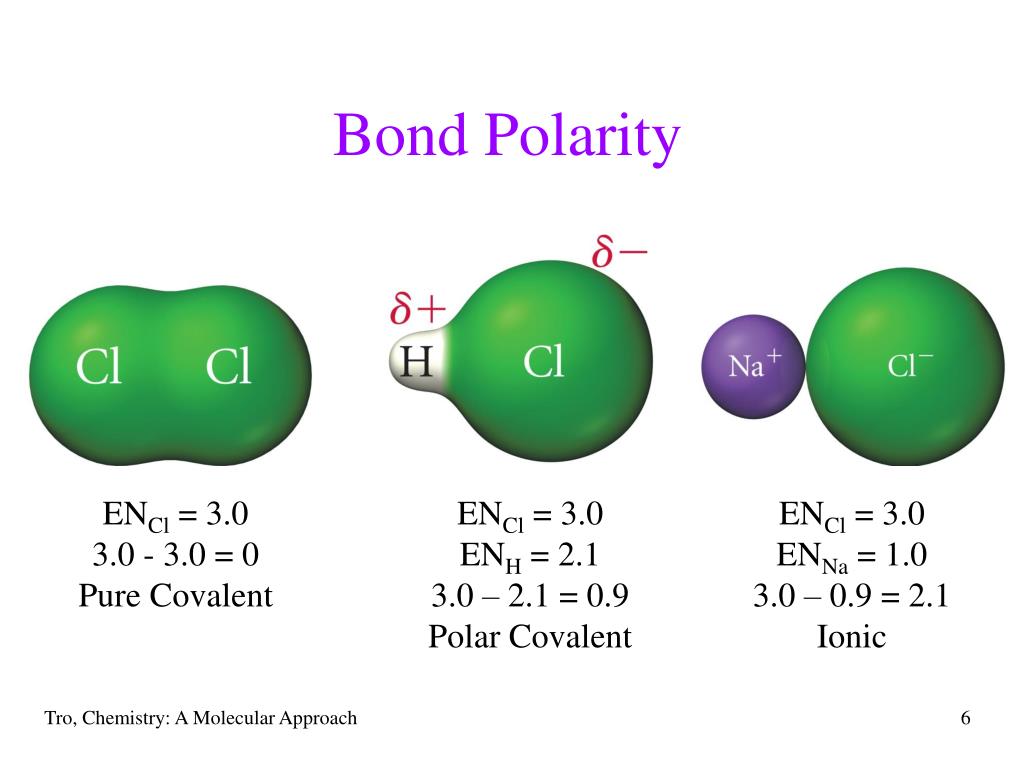
Ppt Polar Bonds And Molecules Powerpoint Presentation, Free Download 587
Common solvents arranged from the least polar to the most polar. Solvent Relative Polarity; hexane 0.009 p-xylene 0.074 toluene 0.099 benzene 0.111 ether 0.117 methyl t-butyl ether (MTBE). acetone 0.355 dimethylformamide (DMF) 0.386 t-butyl alcohol 0.389 sulfolane 0.41 dimethylsulfoxide (DMSO) 0.444 acetonitrile 0.46 nitromethane 0.481.
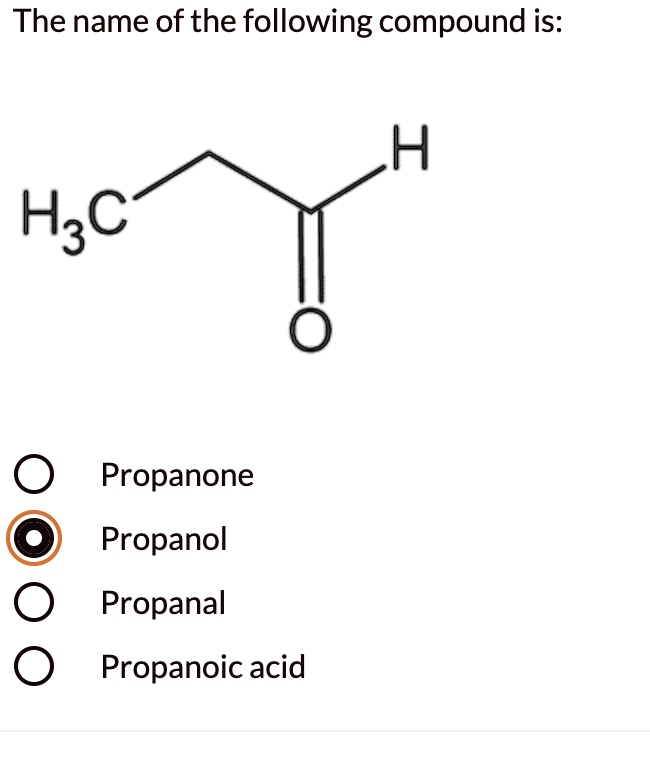
SOLVED The name of the following compound is H3C Propanone Propanol
- Techiescientist Is Acetone Polar or Nonpolar? Acetone is an organic compound with its chemical formula (CH3)2CO. It is classified as the simplest ketone. It exists as a colorless volatile liquid. It has a characteristic odor and flammable in nature. Many students may have a question regarding whether acetone is polar or not.
GCSE Chemistry C3 Organic Chemistry Revision Cards in GCSE Chemistry
217-218 °C Alfa Aesar: 218 °C Food and Agriculture Organization of the United Nations 1-Phenyl-1-propanone: 218 °C OU Chemical Safety Data (No longer updated) More details: 217-218 °C Alfa Aesar A15140: 218 °C Oakwood: 8 °C / 84 mmHg (68.4982 °C / 760 mmHg) FooDB FDB010567 218 °C Sigma-Aldrich SIAL-61074: 218 °C Oakwood 094675

VSEPR, Polarity, and Bonds YouTube
Acetone ( 2-propanone or dimethyl ketone) is an organic compound with the formula (CH3)2CO. [22] It is the simplest and smallest ketone ( >C=O ). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory.

[Solved] Why bond energy of acetone is more though it is 9to5Science
Polarity of Solvents. Water Acetic Acid Ethyleneglycol Methanol Ethanol Isopropanol Pyridine Acetonitrile Nitromethane Diehylamine Aniline Dimethylsulfoxide Ethylacetate Dioxane Acetone Dicholoroethane Tetrahydrofuran Dicholoromethane Chloroform Diethylether Benzene Toluene Xylene Carbontetrachloride Cyclohexane Petroleum ether Hexane Pentane.

Ch3Oh Lewis Structure Geometry Hybridization And Polarity itechguides
Unlike polar bonds, non-polar bonds share electrons equally. A bond between two atoms or more atoms is non-polar if the atoms have the same electronegativity or a difference in electronegativities that is less than 0.4. An example of a non-polar bond is the bond in chlorine. Chlorine contains two chlorine atoms.

Découvrir 117+ imagen propanone formule fr.thptnganamst.edu.vn
∙ 11y ago Study now See answer (1) Best Answer Copy Propane itself is non polar, but the presence of the ketone group (C=O) in propanone makes it a polar molecule (oxygen has partial -ve.

In this video we are going to determine the polarity of CH3OH molecule
Acetone contains a polar C=O double bond oriented at about 120° to two methyl groups with nonpolar C-H bonds. The C-O bond dipole therefore corresponds to the molecular dipole, which should result in both a rather large dipole moment and a high boiling point. Thus we predict the following order of boiling points:
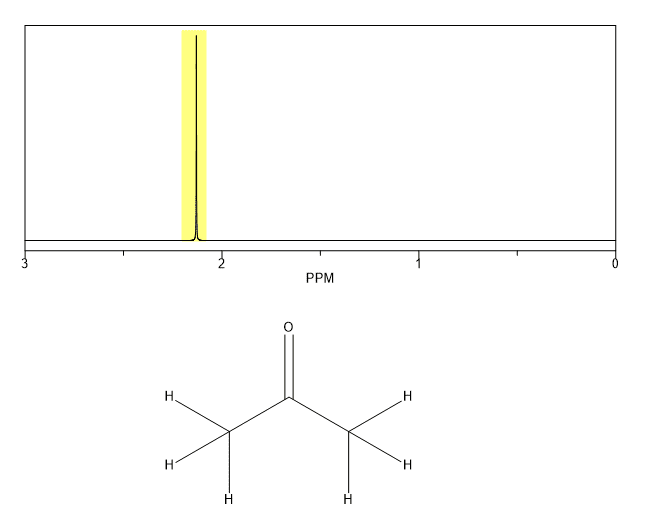
Propanone Proton NMR Equivalent Protons
Propanone is SLIGHTLY POLAR and so can dissolve other polar substances-Propanone is LESS POLAR than water-this allows a RESOLUTION resolution between the pigment and the TLC plate as the pigments will dissolve in the propanone.

Lewis Structure Of 1 Propanol
However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than alcohols. Then again, acetone (and other carbonyl containing solvents) are, indeed, poor solvents when using strong bases due to their relatively high acidity.

Propanone (Acetone)
5. "Borderline" Polar Aprotic Solvents Have Small Dipole Moments And Low (<10) Dielectric Constants. These solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). Since they have intermediate polarity they are good "general purpose" solvents for a wide range of reactions.

Question Video Determining Whether Some Common Simple Molecular
The carbonyl group is rather polar, however, since the difference between the electronegativities of carbon (2.5) and oxygen (3.5) is rather large, and there are usually no other dipoles in an aldehyde or ketone molecule to cancel the effect of C==O.. Like other ketones, acetone is mainly useful as a solvent, and you may have used it for.

SN2 or SN1 polar aprotic and polar protic solvents a Organic Chemistry
Propanone is normally written CH 3 COCH 3. Notice the need for numbering in the longer ketones. In pentanone, the carbonyl group could be in the middle of the chain or next to the end - giving either pentan-3-one or pentan-2-one.. Both aldehydes and ketones are polar molecules because of the presence of the carbon-oxygen double bond. As well.
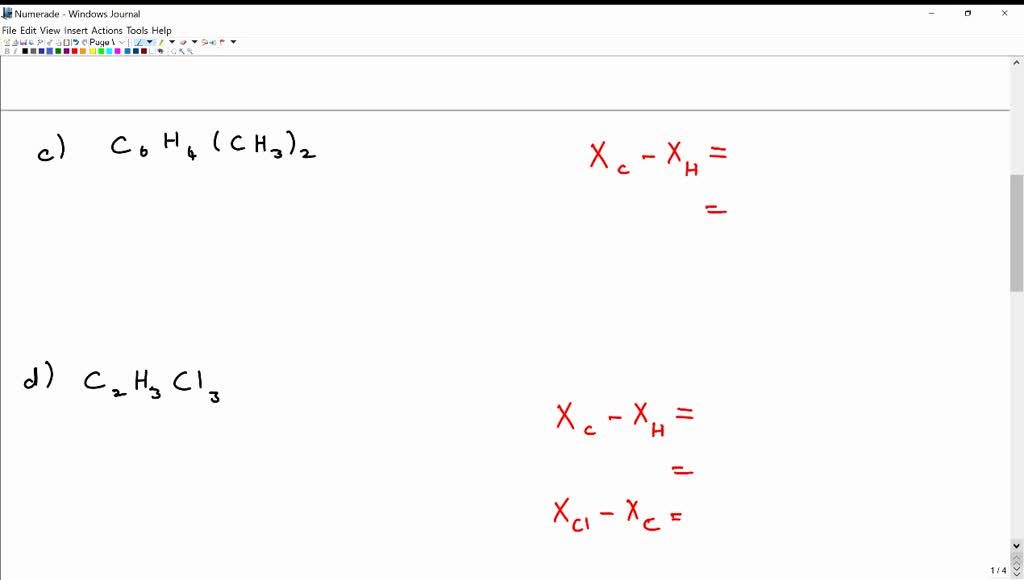
SOLVEDPredict whether each of the following molecules is likely to be
Propanone is very soluble in water because а. it is non-polar b. the dipole-dipole interactions are weak between propane and water С. it can form hydrogen bond with water molecule d. water is a polar solvent Chemistry: The Molecular Science 5th Edition ISBN: 9781285199047 Author: John W. Moore, Conrad L. Stanitski